If a solid substance is added to a liquid, the solid may dissolve forming a solution.
Liquids and gases may also dissolve in liquids. In that situation, the liquid is a solvent while the dissolving substance is called a solute. Sometimes, solids and liquids may not dissolve in a given liquid. The substances are said to be insoluble. The combination is called a mixture. The constituents of the mixtures can be separated by physical means. Particular methods are used in the separation depending on the properties of the constituents. Methods of separating mixtures into their components are important because pure substances are needed for industrial processes.
Separation techniques
Chemists have developed many different methods of separation of mixtures from simple or complex compounds. Methods used depend on what is in the mixture and properties of the substances present. Also depends on whether the substances to be separated are solid, liquid or gas.
Some methods of separating mixtures are:
Filtration separates a solid from a liquid using a filter paper supported in a funnel. Decanting also separates a solid from a liquid. However, in decanting, the mixture is left to settle. The liquid is then carefully poured off the solid.
A centrifuge separates a solid and a liquid by spinning a tube containing the mixture in a circle at several thousands of revolutions per minute. The solid is forced to the bottom of the tube allowing the liquid to be decanted easily.
Simple distillation is similar to fractional. The process involves the evaporation of a liquid from a mixture of a liquid and a solid followed by condensation of the vapour. The condensed liquid is called the distillate. For example sea water on distillation will produce pure water as the distillate and sea salt as a residue.
v. Fractional distillation separates mixtures of liquids (fraction) into their components. Boiling the mixtures causes the components to vaporise in succession – the liquid with the lowest boiling point vaporising first. The vapours are condensed in a water-cooled condenser and collected as distillates. For example, wine on distillation will produce two distillates, ethanol (alcohol) and water, in that order.
vi. Crystallisations occur when a hot, saturated solution is allowed to cool. The crystals that form will be the pure solid component if solvent is the only other component. Decanting the remaining solution allows the crystals to be dried on a tissue and so obtained pure.
vii. Paper chromatography is the process of separating a mixture of solids, in solution, on a sheet of paper.
The table below summarises the types of separation techniques used in industry and in the laboratories.
|
Substance |
Deposit |
Result |
Means of Separation |
|
Water |
Sodium Chloride |
Solution |
Evaporation |
|
Water |
Copper Sulphate |
Saturated Solution |
Crystallization |
|
Water |
Soil |
Mixture |
Filtration |
|
Sulphur |
Iron |
Mixture |
Magnetization |
|
Iodine |
Sodium Chloride |
Mixture |
Sublimation |
|
Common Salt |
Water |
Solution |
Distillation |
|
Water |
Alcohol |
Solution |
Fractional Distillation |
|
Paraffin |
Water |
Mixture |
Decanting |
|
Colour |
Colour |
Solution |
Chromatography |
|
Milk |
Butter |
Mixture |
Centrifugation |
When the substances are separated, the final products approach the original purity of the original substance.
Substances are made up of some basic substances called “elements”. Each element is composed of its own kind of particles called “atoms”. For example, aluminium is an element which is made up of only aluminium atoms. An Element cannot be broken any further. Elements can be classified as “metals and nonmetals”.
The atoms of all elements are extremely small to be seen. The smallest atom known is hydrogen with diameter 7×10-8mm. atoms of different elements have different diameters as well as different masses.
Chemist use shorthand symbols to label the elements and their atoms. The symbol consists of one, two or three letters, the first of which must be a capital. For example, C is used for Carbon, Ca for Calcium and Cl for Chlorine. Some symbols seem to have no relationship to the name of the element, for example Na for Sodium and Pb for lead. These symbols came from their Latin names Natrium for Sodium and Plumbum for Lead.
To make sense of the material world around us, we need methods for physically separating the many and varied mixtures that we come across. Being able to purify and identify the many substances present in these mixtures not only satisfies our curiosity but is crucial to our well-being and health. A range of physical techniques are available to make the necessary separations. They all depend in some way on a difference in the physical properties of the substances in the mixture.
The most useful separation method for a particular mixture depends on:
• the type of mixture, and
• which substance in the mixture we are most interested in.
Separating heterogeneous mixtures
In some ways these are the easier mixtures to separate. Quite often, just leaving them to stand helps with the separation. This is often the first stage in separating mixtures of immiscible liquids. It is also often used to separate solid-in-liquid suspensions if the particles of solid are large enough. Once the solid has settled to the bottom (sedimented), the liquid can be carefully poured off (this is called decantation).
A more generally useful method than decantation for separating solids from liquids is filtration. Here the insoluble material is collected as a residue on filter paper. Filtration is useful because both phases can be obtained in one process. The liquid phase is collected as the filtrateThe process can be speeded up by using a vacuum pump to ‘suck’ the liquid through the filter paper in a Buchner funnel and flask.
Various large-scale filtration methods are used in industry. Perhaps the most useful of these are the filter-beds to purify water for household use.
Table 2.5 Methods of separating substances from mixtures
|
Type of mixture |
Mixture |
Method of separation |
|
Heterogeneous |
Solid + solid (powdered mixture) |
use some difference in properties, e.g. density, solubility, sublimation, magnetism |
|
Suspension of solid in liquid |
filtration or centrifugation |
|
|
Liquid + liquid (immiscible) |
use a separating funnel or decantation |
|
|
Homogeneous |
Solution of solid in liquid |
to obtain solid: use evaporation (crystallisation) to obtain liquid: use distillation |
|
Two (or more) liquids mixed together (miscible) |
fractional distillation |
|
|
Solution of two (or more) solids in a liquid |
chromatography |
Another means of separating an insoluble solid from a liquid is centrifugation where the mixture is spun at high speed in a centrifuge. Here, it is no longer the force of gravity on the solid particles that causes settling. Instead, there is a huge centrifugal force acting on the particles due to the high-speed spinning of the samples. This causes the solid to be sedimented at the bottom of the centrifuge tube. The liquid can be decanted off carefully.
Mixtures of two immiscible liquids can be separated if the mixture is placed in a separating funnel and allowed to stand. The liquids separate into different layers. The lower, denser layer is then ‘tapped’ off at the bottom. This type of separation is useful in industry. For example, at the base of the blast furnace the molten slag forms a separate layer on top of the liquid iron. The two can then be ‘tapped’ off separately. The method is also very useful in organic chemistry as part of a process called ‘solvent extraction’.
The separation of a solid from a mixture of solids depends largely on the particular substance being purified. Some suitable difference in physical properties needs to be found. Usually it helps if the mixture is ground to a powder before any separation is attempted.
1. Separations based on differences in density
‘Panning’ for gold is still carried out in the search for new deposits. In Amazonia, river-beds are mechanically sifted (‘vacuum-cleaned’) to collect gold dust. These methods depend on the gold dust being denser than the other substances in the river sediment. This type of method is also used in purifying the ores of zinc and copper, although in these cases the metals are less dense than the ores and so float on the surface.
1. The mixture of immiscible liquids settles into two layers, as the liquids do not mix
2. The tap is opened to let only the bottom layer run info the beaker
3. The tap is closed and the beaker is changed. The tap is opened to let the top layer run out
 |
| A separating funnel can be used to separate two immiscible liquids. |
2. Separations based on magnetic properties
Magnetic iron ore can be separated from other material in the crushed ore by using an electromagnet. In the Amazonian gold diggings, magnets are used to clean away iron-containing, red-brown dust from the powdered gold. In the environmentally and economically important processes of recycling metals, iron objects can be picked out from other scrap metal using electromagnets.
3. Separations based on differences in solubility
One very useful way of separating a soluble substance from a solid mixture is as follows. The mixture is first ground to a powder. A suitable liquid solvent is added. The solvent must dissolve one of the solid substances present, but not the others. The solvent is often water, but other liquids can be useful. The mixture in the solvent is then warmed and stirred. Care must be taken at the warming stage when using solvents other than water.
The warm mixture is then filtered. This gives the insoluble substances as a residue on the filter paper, which can be dried. The soluble substance is in the liquid filtrate. Dry crystals can be obtained by evaporation and crystallisation.
The gold prospectors in Brazil and Zimbabwe still use an immensely dangerous version of this method to extract the gold from other substances. The solvent they use is mercury, which dissolves the gold. The gold is then recovered from solution by evaporating off the mercury with a blowtorch.
The unwanted residues, contaminated with mercury, are thrown into the rivers. Damage to the environment from this activity is very likely because mercury is poisonous to living things.
4. Separations based on sublimation
A solid that sublimes can be separated from others using this property.
Separating homogeneous mixtures
The separation of homogeneous mixtures is often slightly more complicated because there is no physical separation of the phases in the original mixture. The methods of separation usually depend on solubility properties or on differences in boiling point.
For example, Ammonium chloride can be separated from a mixture because it sublimes. The crystals condense on the cooled surface.
| Separation of Ammonium chloride |
Separating a solid from solution in a liquid can be carried out by evaporation or crystallisation. Evaporation gives only a powder, but crystallisation can result in proper crystals. Both processes begin by evaporating away the liquid but, when crystals are needed, evaporation is stopped when the solution has been concentrated enough. Figure 2.10 shows how this can be judged and done safely. The concentrated solution is allowed to cool slowly. The crystals formed can then be filtered off and dried.
Separating a liquid from a solution is usually carried out by distillation. The boiling point of the liquid is usually very much lower than that of the dissolved solid. The liquid can easily be evaporated off in a distillation flask. It is condensed by passing it down a water-cooled condenser, and then collected as the distillate.
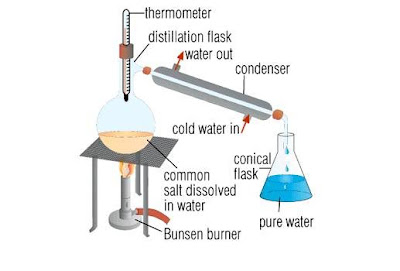 |
| Separation by distillation – An evaporation method |
While the solvent is evaporating, dip a glass rod into the solution from time to time. When small crystals form on the rod, take the solution off the water bath and leave it to cool.
This method should not be used if the solvent is flammable. Instead, use an electrical heating element and an oil or water bath.
Separating the liquids from a mixture of two (or more) miscible liquids is again based on the fact that the liquids will have different boiling points. However, the boiling points are closer together than for a solid-in-liquid solution and fractional distillation must be used.
For example, ethanol boils at 78 °C whereas water boils at 100°C. When the mixture is heated, ethanol and water vapours enter the fractionating column. Glass beads in the column provide a large surface area for condensation. Evaporation and condensation take place many times as the vapours rise up the column. Ethanol passes through the condenser first as the temperature of the column is raised above its boiling point. Water condenses in the column and flows back into the flask because the temperature of the column is below its b.p. of 100°C.
 |
| Separating a mixture of ethanol (alcohol) and water by fractional distillation |
The temperature on the thermometer stays at 78 °C until all the ethanol has distilled over. Only then does the temperature on the thermometer rise to 100°C and the water distils over. By watching the temperature carefully, the two liquids (fractions) can be collected separately.
Fractional distillation is used to separate any solution containing liquids with different boiling points. It can be adapted as a continuous process and is used industrially to separate:
• the various fractions from crude oil,
• the different gases from liquid air .
Solubility
The solubility of solids in liquids
Probably the most important and common examples of mixtures are solutions of solids in liquids.
Such a solution is made up of two parts:
• the solid that dissolves is known as the solute,
• the liquid in which it dissolves is called the solvent.













